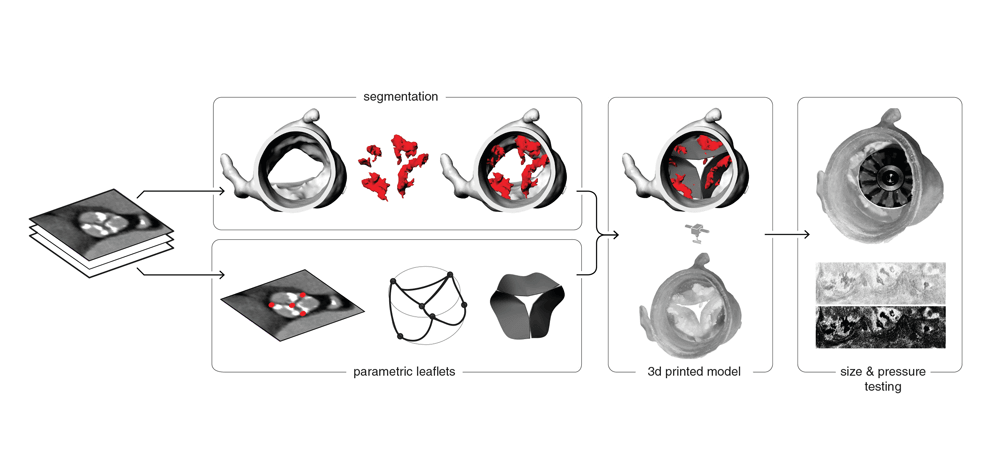
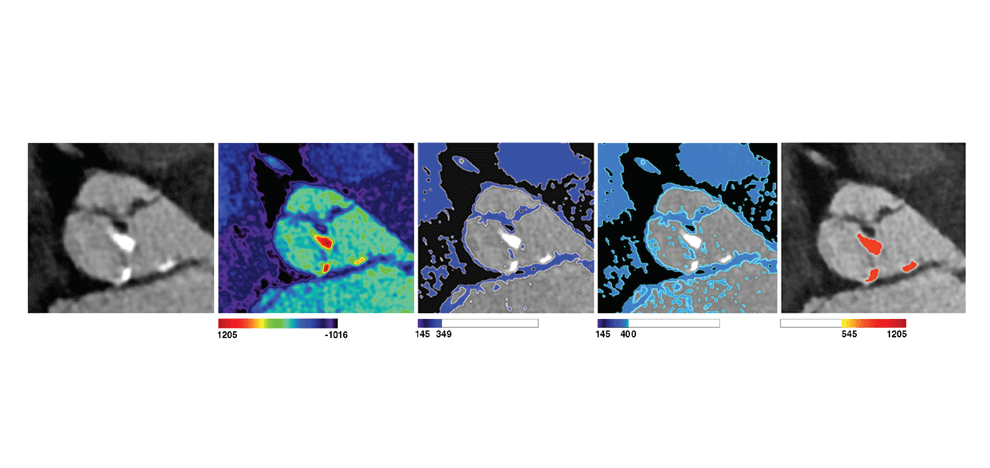
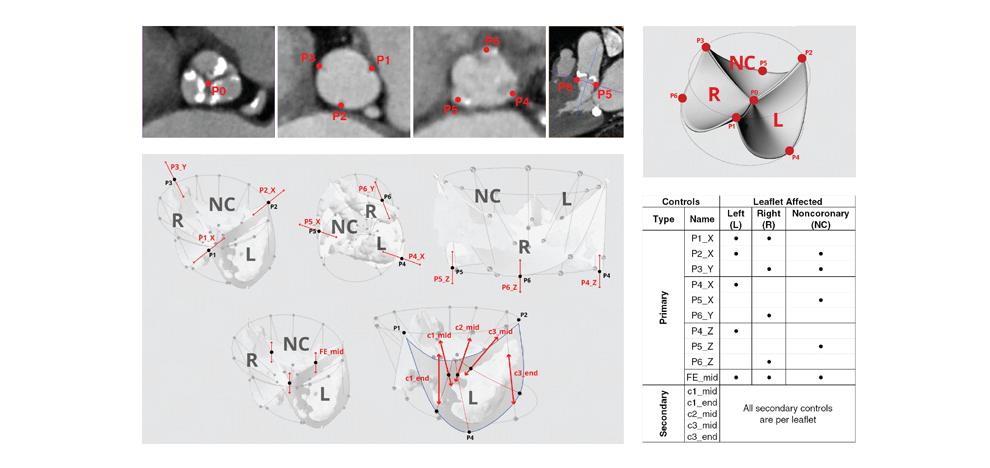
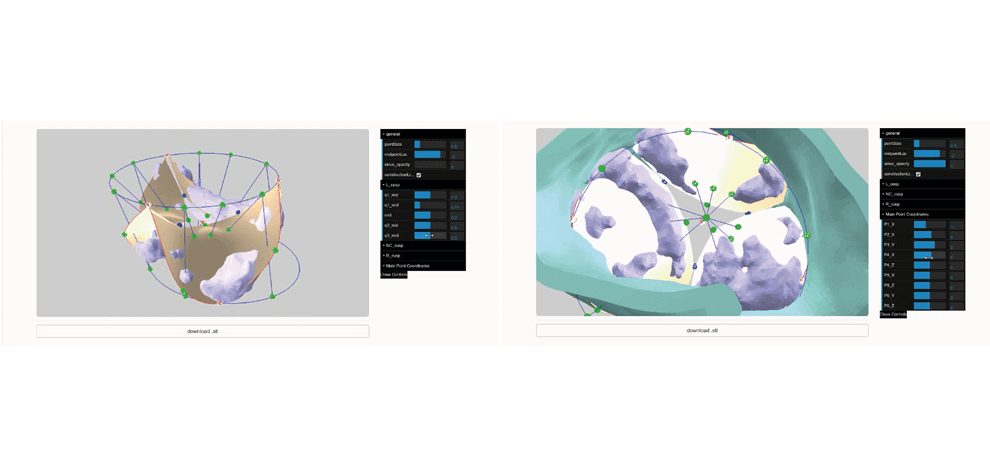
TAVR Simulation
Background. Successful transcatheter aortic valve replacement (TAVR) requires an
understanding of how a prosthetic valve will interact with a patient’s anatomy in advance of
actual deployment. To improve this understanding, we developed a benchtop workflow that
allows for testing of physical interactions between prosthetic valves and patient-specific aortic
root anatomy, including calcified leaflets, prior to actual prosthetic valve placement.
Methods. This was a retrospective study of 30 patients who underwent TAVR at a single high
volume center. By design, the dataset contained 15 patients with a successful annular seal
(defined by an absence of paravalvular leaks) and 15 patients with a sub-optimal seal (presence
of paravalvular leaks) on post-procedure transthoracic echocardiogram (TTE). Patients received
either a balloon-expandable (Edwards Sapien or Sapien XT, n=15), or a self-expanding
(Medtronic CoreValve or Core Evolut, n=14, St. Jude Portico, n=1) valve. Pre-procedural
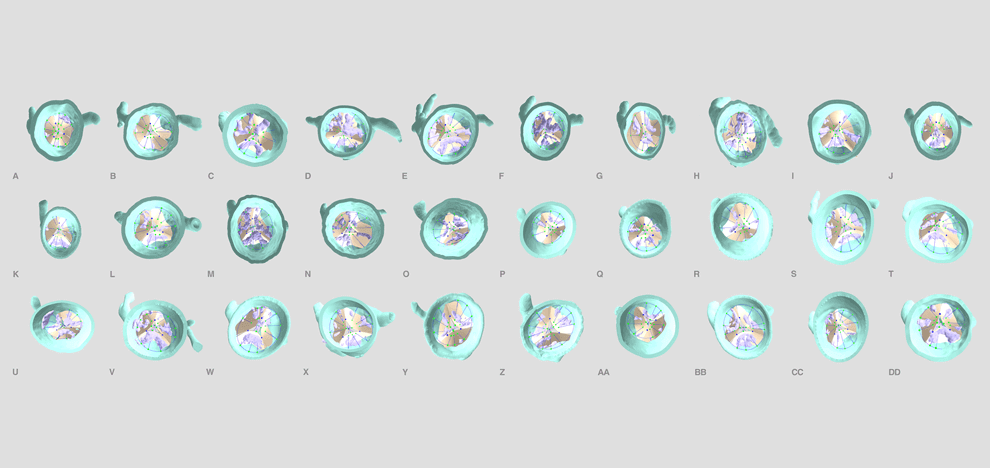
computed tomography (CT) angiograms, parametric geometry modeling, and multi-material 3D
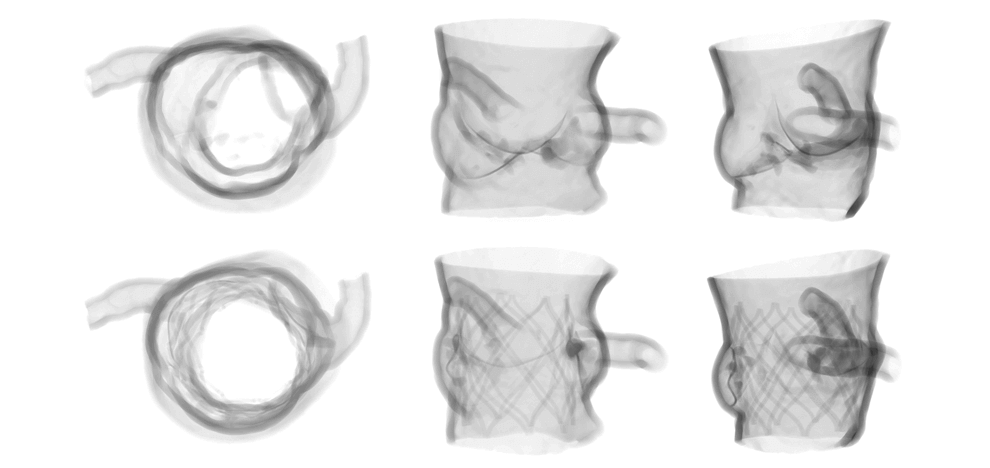
printing were utilized to create flexible aortic root physical models, including displaceable
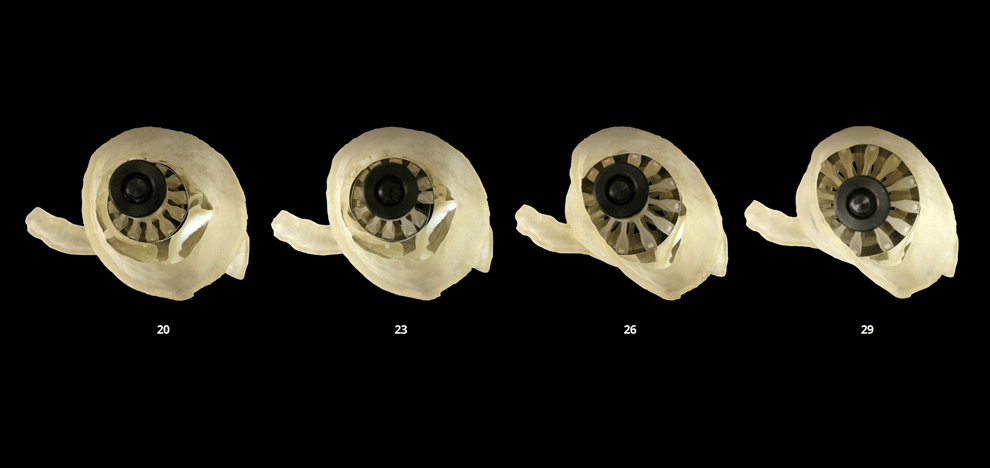
calcified valve leaflets. A 3D printed adjustable sizing device was then positioned in the aortic
root models and sequentially opened to larger valve sizes, progressively flattening the calcified
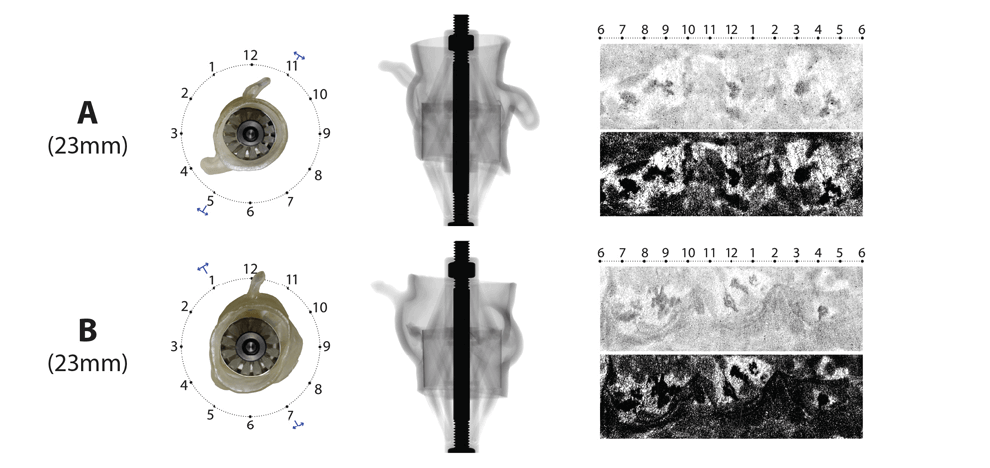
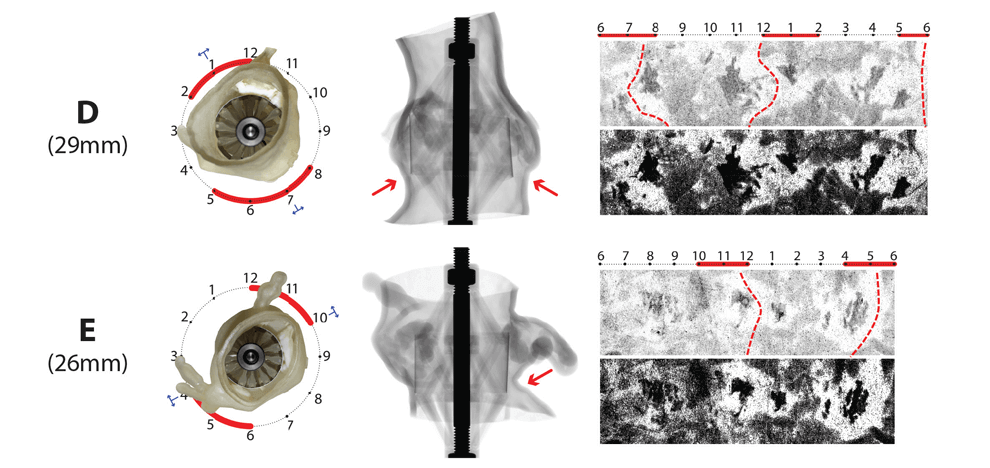
leaflets against the aortic wall. Optimal valve size and fit were determined by visual inspection
and quantitative pressure mapping of interactions between the sizer and models.
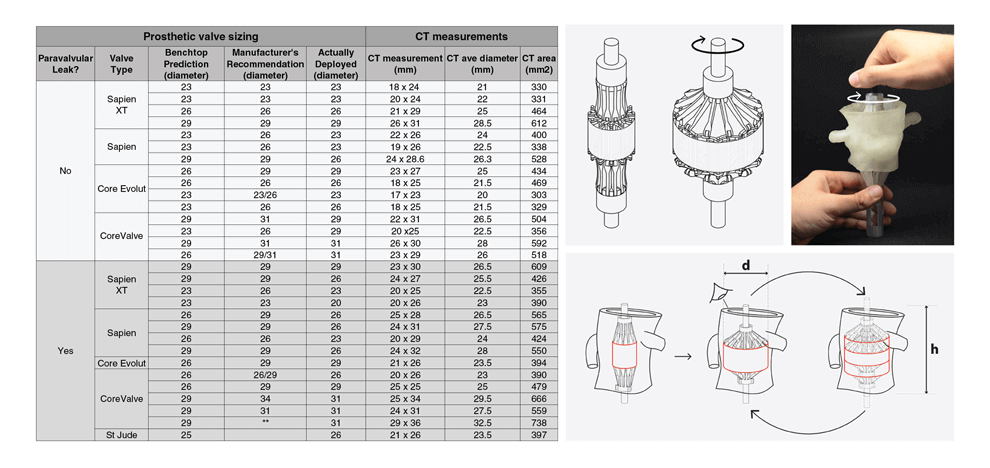
Results. Benchtop-predicted “best fit” valve size showed a statistically significant correlation
with gold standard CT measurements of the average annulus diameter (n=30, p < 0.0001
Wilcoxon matched-pairs signed rank test). Adequateness of seal (presence or absence of
paravalvular leak) was correctly predicted in 11/15 (73.3%) patients who received a balloonexpandable
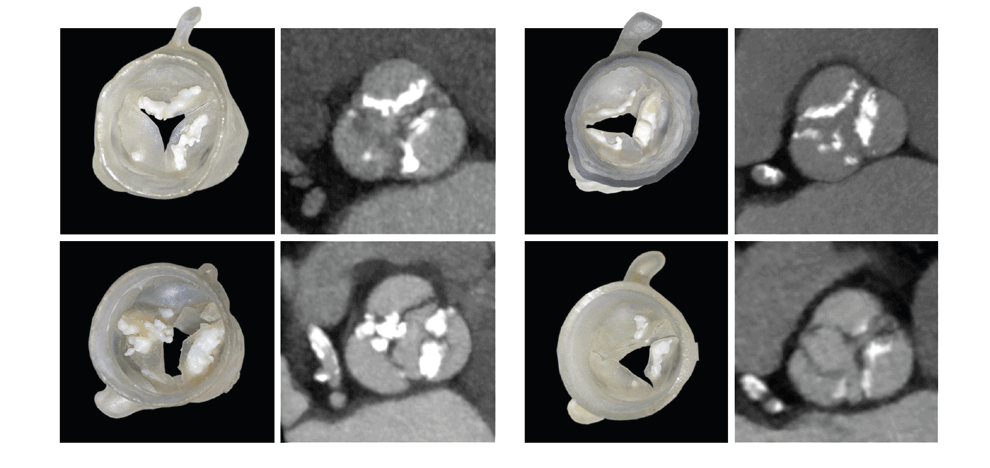
valve, and in 9/15 (60%) patients who received a self-expanding valve. Pressure
testing provided a physical map of areas with an inadequate seal; these corresponded to areas of
paravalvular leak documented by post-procedural transthoracic echocardiography.
Conclusion. We present and demonstrate the potential of a workflow for determining optimal
prosthetic valve size that accounts for aortic annular dimensions as well as the active
displacement of calcified valve leaflets during prosthetic valve deployment. The workflow’s
open source framework offers a platform for providing predictive insights into the design and
testing of future prosthetic valves.
The manuscript was published in the Journal of Cardiovascular Computed Tomography and can be found here, in addition to the web app, github repository, and sizing hardware.